Q1: Can the GFP sequences for HPV16 and HPV18 be provided?


GFP is conventional, but after codon optimization, the specific sequence is not convenient to provide externally
Q2: In neutralization assays, after 72 hours, does the medium need to be changed during detection? Does the medium turning yellow affect cell state?


The test will be conducted 72 hours later, and no liquid change is required in between. A slight yellowing is normal and will not affect the cell state. At the same time, it should be noted that the initial number of inoculated cells should not be too large. It is best to inoculate according to the number of cells we recommend
Q3: For neutralization assays with GFP pseudovirus, can I finally permeabilize and stain GFP for flow cytometry detection?


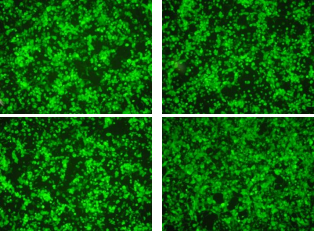
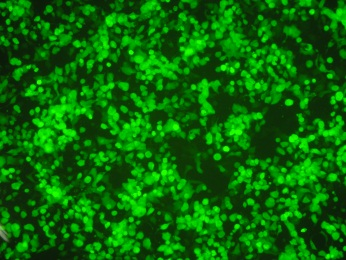
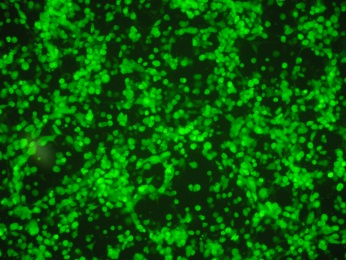
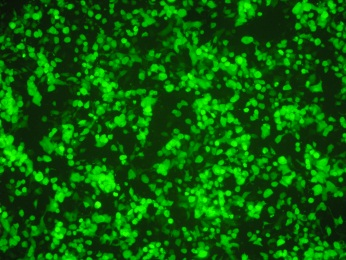
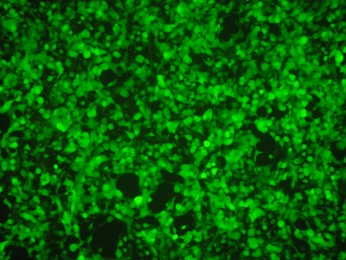
Flow cytometry is also acceptable, as long as it can detect the amount of GFP, which is directly proportional to the pseudovirus infection.
Q4: Is it only for L1 experiments, or can it be used for both L1 and L2 experiments simultaneously?


Pseudoviruses have both L1 and L2 proteins on their surfaces, allowing for relevant neutralization experiments on L1 as well as L2.
Q5: Is there a Luciferase (Luc) HPV pseudovirus?


None
Q6: Why is there no Luciferase (Luc) pseudovirus?


Because gfp is sensitive enough and its cost is lower; The pseudovirus of Luciferase is very costly to use and also requires a substrate
Q7: For pseudovirus harvest, is only the cell supernatant collected? Is cell lysis necessary?


The general process is: transfection - removal of supernatant - lysis - collection of supernatant
Q8: Planning to use HPV pseudovirus for neutralization assays. Generally, after vaccination, human serum is collected. At what dilution range is the serum typically used for neutralization assays? Has there been testing for an approximate range?


The general process is: transfection - removal of supernatant - lysis - collection of supernatant
Q9: Can our HPV pseudovirus infect HeLa cells?


We don't have experimental data, so we're not sure either.
Most HPV pseudovirus infection experiments use 293 cells, and the effect of ordinary 293 cells may not be good. Only 293FT or 293TT cells must be used.
Q10: For HPV16 and HPV18 pseudoviruses, after lysis with virus lysis buffer, can E6 and E7 antigens be detected?


There is no E6. And E7



 Save
Save










 Phone
Phone
 Contact Us
Contact Us
 QQ
QQ
 QR Code
QR Code
 Scan the QR code
Scan the QR code